Wet / Green Phosphoric Acid and Spent Sulphuric Acid damage repairs
INOX Shanghai (CN) & INOX Surface Treatment (NL) successfully completed many repairs of stainless steel chemical tankers which suffered cargo tank damages due to Phosphoric or Sulphuric acid cargo. The concerned stainless steel cargo tanks were extreme affected by Wet/Green Phosphoric Acid cargo or Spent Sulphuric Acid cargo.
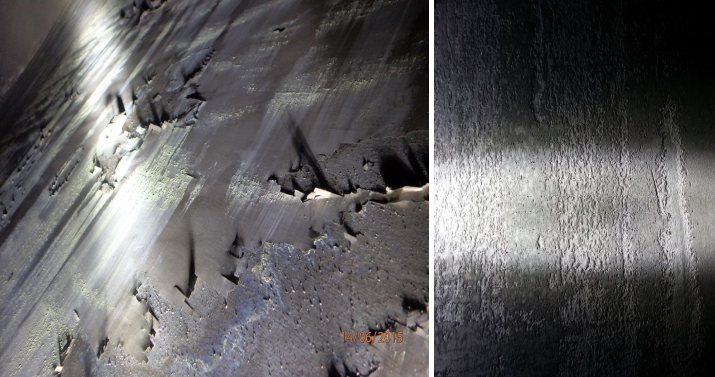
During our inspections we observed rough surfaces (some cases even elephant skin and flakes, especially on the HAZ) and grey/black dust contamination.
The corrosion to austenitic stainless steel occurring due to carriage of Wet / Green Phosphoric Acid or Spent Sulphuric Acid is a very complex phenomenon. Both acids are perhaps the most aggressive and difficult of all chemicals transported in a stainless steel chemical tanker. The corrosion is affected by various impurities and temperature.
Clarification of Acid Corrosion on stainless steel:
Wet / Green Phosphoric Acid and Spent Sulphuric Acid can be aggressive towards stainless steel, depending amongst the combination of
-
Grade of stainless steel
-
Range of impurities that the acid contains
-
Temperature
Chlorides, fluorides and sulfuric acid impurities increase the risk of corrosion, along with high temperatures. The Chlorides and Fluorides determine the corrosivity as they are corrosion accelerators. The percentage of Fluorides and Chlorides differs from country to country where the raw stock comes from.
Most acids can be safely carried at ambient temperature in stainless steel. Reducing (passive surface becomes active surface) acids, like Wet / Green Phosphoric Acid or Spent Sulphuric Acid are theoretically unsuitable for carriage in stainless steel tanks.
Note: when temperature linear increases, aggressively of acids will increase exponential!
The sediment in Wet / Green Phosphoric Acid will exclude oxygen from the stainless steel and can cause active area s. The result of this “reaction” is a black powder and elephant skin on stainless steel. Especially the HAZ (Heat Affected Zones) are very sensitive for this reaction, which expresses by very high roughness and even flaking.
Rough surfaces (Ra > 3,5 µ) exhibit deep grooves where chloride ions can accumulate and destroy the passive layer, thereby initiating corrosion attack.
To reduce the possibility for these corrosion attacks, fine polished finishes with Ra values < 3,5µ (micron) will generally exhibit clean-cut surfaces, with few sites where chloride ions can accumulate.
The advantage of a smooth polished finish is a better corrosion resistance but will never give guarantee for a corrosion free stainless steel surface in the future !
Cleaning advice:
Proper cleaning prior and after these acid cargoes requires special attention in order to avoid contamination.
Before loading:
Chemical passivation prior loading is an option to enrich the chromium oxide layer (passive layer)
Dry tanks are crucial before loading, to avoid any reaction between the acids and water / condense
After discharging:
- Wet / Green Phosphoric Acid:
Directly pre-wash with fresh water (no circulation, pump out same time), neutralization with seawater and pump out, finally fresh water rinse to remove all chlorides from tank surface. This cleaning procedure is crucial to avoid elephant skin and or pitting corrosion.
After final cleaning with fresh water (low chloride), tanks need to be passivated in order to restore the passivity of the stainless steel.
- (Spent) Sulphuric Acid:
Directly wash down the acid with overload of water (sea water / freshwater) with as tank wash machines as fire hoses as via dropline, to decrease as quick as possible the concentration of the acid and avoid as much as possible the exothermic reaction between H2SO4 and flushing water. Fresh water washing afterwards to remove all chlorides from tank surface is necessary. This cleaning procedure is crucial to avoid elephant skin and or pitting corrosion.
- Passivation
After final cleaning with fresh water (low chloride), tanks need to be passivated in order to restore the passivity of the stainless steel.
- Storage
Storage of slobs / waste waters: Please note that mixtures of these acids with seawater/fresh water can cause elephant skin and or pitting corrosion!
INOX Shanghai (CN) & INOX Surface Treatment (NL) performed repairs & surface treatments:
- Grinding and Polishing, removal of high surface roughness and grey/black residue
- Surface roughness measurements
- Degreasing of surfaces by circulation
- Pickling/passivating by circulation
- Oxilyser 3 passivity tests and Ferroxyl test ( free / embedded iron )

100% clean and smooth surface roughness Ra value < 3.5 µ (micron)
Achieved end result by INOX Shanghai (CN) & INOX Surface Treatment (NL) after repairs:
- 100% clean and smooth surface roughness Ra value < 3.5 µ (micron)
- Passivity (by electronic oxilyser test equipment) 75 up to 96
- Cargo tanks suitable for wall wash sensitive cargoes.

